Accurate. Safe. Efficient. Proven.
Augmented Reality Spine Surgery: The Future is Here
AR navigation puts spine surgery back where it belongs: in your hands, and in your eyes.
*Molina CA, et al. J Neurosurg Spine. 2020 Oct 30:1-9.
Your skills, Your workflow, Your sight, augmented.
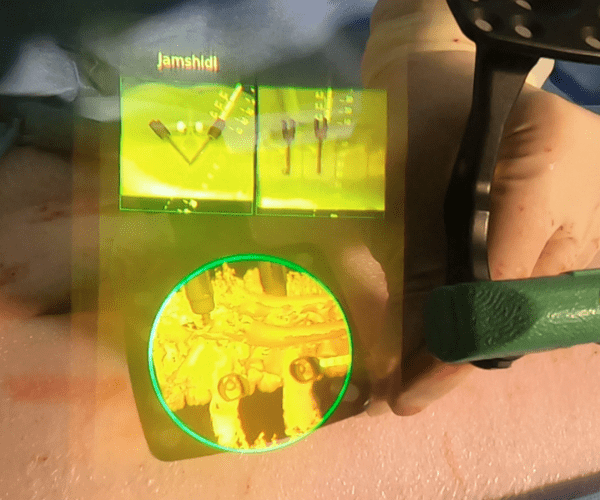
X-ray vision doesn’t change how you perform surgery. It’s an extension of what you do best.
We’re here to augment your:
- Accuracy: Clinically proven for peace of mind.
- Safety: X-ray vision without the actual x-ray.
- Efficiency: We fit into and accelerate your workflow.
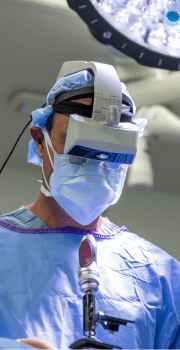
“Augmented reality has changed the way I practice spine surgery.
I’ve seen exceptionally good results with it. I think it makes me a more accurate, more efficient surgeon. And I think that ultimately leads to shorter surgical times and better results for our patients.”
Frank M. Phillips, MD
Ronald DeWald Endowed Professor of Spinal Deformities, Rush University Medical Center

Meet X2
We’re on a mission to eliminate barriers to navigation adoption. X2 is easy to use, and integrates seamlessly into your workflow.- No attention shift
- Open platform
- Intuitive 3D user interface
- Short learning curve
- Preop or Intraop registration
Simplify Your Surgery
Your eyes on the patient. Not on a screen.
Legacy navigation forces you to look away from your patient while operating. That’s not how you trained. That’s not how you perfected your craft.
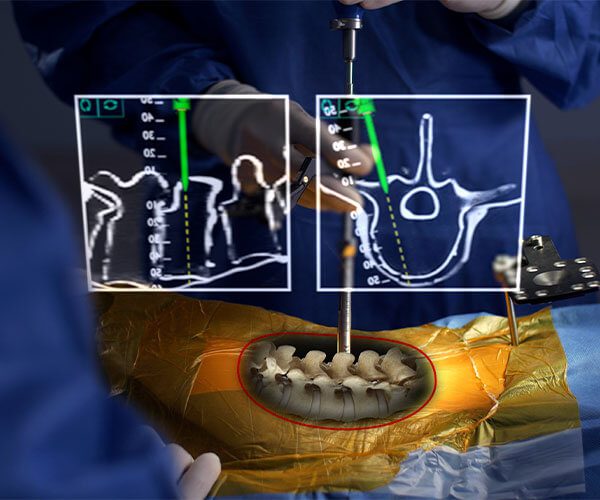
Augmented reality combines the benefits of navigation with the ease and utility of open surgery. You can look directly at the patient and visualize their anatomy in 3D.
No looking away to a distant screen. No line of sight interruptions.
AR augments what’s most important: your hands, your eyes, and your talent.
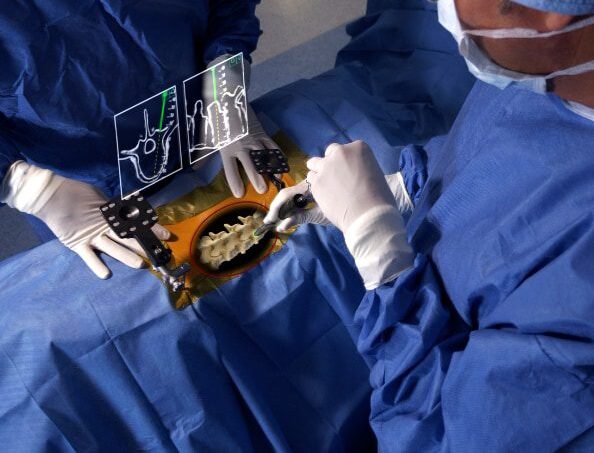
Procedural Applications of AR
Augmedics is broadly applicable across a wide range of procedures. xvision is FDA approved for open or percutaneous spine procedures for C3 to pelvis.
Open, MIS, or Percutaneous
Cervical (C3-C7), Thoracic, Lumbar, or Pelvis
Single- or multi-level
Degen, Trauma, Revision, Complex/Deformity
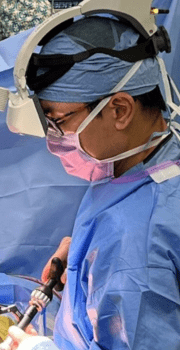
“I can optimize my efficiency and accuracy in surgery.
Augmented reality brings a number of data points into our surgical field, so I can optimize my efficiency and accuracy in surgery. As surgeons, we are constantly processing and interpreting numerous data points… AR helps bring that data into my visual field to implement the plan that I am trying to execute.”
John H. Shin, MD
Chief of Spine Surgery, Penn Medicine, University of Pennsylvania Health System
Surgical Navigation Without Compromise
Accuracy or efficiency? Speed or safety? Less radiation or lower cost?
Other enabling technologies force you to make tradeoffs. And that compromises your work.
We believe all surgeons and patients deserve access to the proven benefits of surgical navigation without compromise.
Peer-Reviewed. Proven. Potential Realized.
Dive into the data on augmented reality surgical navigation.
Explore published studies
xvision has consistently demonstrated 97-100% accuracy of pedicle screw placement across multiple patient studies.
A game changer for MIS
Rush University’s Dr. Frank Phillips on how augmented reality navigation is changing the game for minimally invasive spine surgeons.
The forefront of surgical innovation
UC Davis’ vice chair for surgical innovation, Dr. Safdar Khan, on how AR is taking spine surgery to the next level.
Schedule a Live Augmented Reality Spine Surgery Demo at Your Practice
Get x-ray vision.
Contact Us for an In-Person Demo